Abstract
Introduction: Despite significant improvement in the outcomes of early treatment lines in myeloma, most patients (Pts) invariably relapse. As triple and quadruple regimens are being introduced at the upfront setting, many Pts are either triple-exposed or triple-refractory early in their disease course. We report here the outcomes of a pre-specified interim analysis of the IPoD-790 clinical trial (NCT04790474), an all oral ixazomib (IXA) pomalidomide (POM) dexamethasone (DEX) (IPd) combination, in Pts with relapsed refractory multiple myeloma (RRMM) previously treated with bortezomib (BTZ) lenalidomide (LEN) and daratumumab (DARA).
Methods: A phase 2, investigator initiated, open-label, single-arm, prospective, multicenter study evaluating the safety, tolerability and efficacy of IPd as a second or third-line combination treatment for Pts with RRMM who progressed after receiving BTZ, LEN and DARA during the first and second treatment lines. IXA is administered twice-weekly during the first 3 cycles. Pts receive IXA: 3 mg days (d) 1, 4, 8, 11 of 21-d Cycles (Cy) 1-3; 4mg d 1, 8, 15 of 28-d Cy 4-24. POM 4mg: d 1-14 Cy 1-3; d 1-21 Cy 4-24. DEX 20mg: d 1,2,8,9,15,16 Cy 1-3; d 1,2,8,9,15,16, 22,23 Cy 4-24. Treatment continues until disease progression (PD) or unacceptable toxicity. After completion of 24 treatment cycles, Pts continue POM DEX as standard of care. The primary endpoint is progression free survival (PFS) according to IMWG criteria.
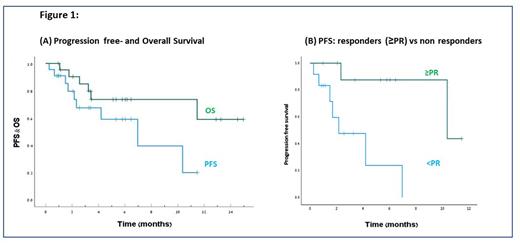
Results: Twenty-two Pts (out of the 60 Pts planned) enrolled between February 2021-May 2022. The median age was 71 (range: 53-88), median time from Myeloma diagnosis to study enrollment was 3.7 years; 58% had high risk FISH cytogenetics (t(4:14), +1q21, del17p); 10% had extramedullary disease at study enrollment; 32%, 9%, 73% and 86% of the Pts were refractory to BTZ, cyclophosphamide, LEN and DARA, respectively. Forty-five percent had previously undergone an auto-transplant. At the interim analysis cutoff date of 28-Jun-2022, after a median follow-up of 5.8 months (mo) (95% CI 4.5-7.1), 13 Pts discontinued the study treatment due to disease progression (PD) (n=9, 41%), death (n=2, 9%, 1 each: COVID-19 and cerebrovascular accident) and withdrawal of consent (n=2, 9%). Eighty-six percent of the Pts had at least one treatment emergent adverse event (TEAE) and 59% had a TEAE grade ≥3. TEAE that occurred in ≥10% include neutropenia 27% (23% ≥Gr 3), thrombocytopenia 27% (14% ≥Gr 3), rash 23%, diarrhea 14%, dyspnea 14%, fatigue 14%, COVID-19 14% and pneumonia 14% (14% ≥Gr 3). No Pts discontinued therapy due to an AE. Overall response rate was 53% (10/19 of evaluable Pts); 2 (10%) complete or very good partial response (CR/VGPR); 8 (42%) partial response (PR); 6 (32%) stable disease; 3 (16%) PD. Median time to response was 0.7 (range: 0.4-6) mo. The median PFS and overall survival (OS) were 6.9 [95% CI 2.0-12.0] and not-reached (NR), respectively [Figure 1A]. Median PFS was 10.4 vs 2.2 mo among Pts who achieved at least a PR vs non-responsive Pts, respectively (p=0.005). [Figure 1B].
Conclusions: IPd combination with twice-weekly ixazomib provided an all-oral safe and efficacious therapy in triple-exposed RRMM Pts, most of whom were refractory to both DARA and LEN. Study enrollment is ongoing.
Disclosures
Cohen:Neopharm: Honoraria; Amgen: Honoraria, Research Funding; Medison: Honoraria; GSK: Honoraria; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Avivi:Novartis: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.